We did a lab using a nail and a chlorine solution. We place the nail inside the solution and let it sit there for 2 to 3 days. Afterwards we can see the chemical reaction taking place. Within 5-10 minutes you can see the rust begin to form. It was a really cool lab.
The next day we isolated the copper from the solution and the nail. The reaction with the iron nail left the copper remaining in the jar with the solution. We rinsed the solution out with hydrochloric acid and distilled water. This helped isolate the copper. The nail seemed to look like it had parts of it melted off. It was really cool.
Monday, December 14, 2015
Wednesday, December 9, 2015
Percent Yield
This was our last day of notes this semester in chemistry. It was probably the easiest lesson we will have all year. Percent yield is basically finding out the percentage of the product that was actually formed. We use the actual yield from a lab and divide it by the theoretical yield of the product and multiply it by 100. It is a fairly easy concept.
https://www.khanacademy.org/science/chemistry/chemical-reactions-stoichiome/limiting-reagent-stoichiometry/a/limiting-reagents-and-percent-yield
https://www.khanacademy.org/science/chemistry/chemical-reactions-stoichiome/limiting-reagent-stoichiometry/a/limiting-reagents-and-percent-yield
Limiting Reagents
This topic is just adding on to the knowledge of what we learned the other day. We compare the yields that we get of the product from the two reactants. The reactant that produces the least amount of product is the limiting reagent. It limits the amount of product that the reaction can produce.
These are some helpful links that I have found.
https://www.chem.tamu.edu/class/majors/tutorialnotefiles/limiting.htm
https://www.khanacademy.org/science/chemistry/chemical-reactions-stoichiome/limiting-reagent-stoichiometry/v/stoichiometry-limiting-reagent
These are some helpful links that I have found.
https://www.chem.tamu.edu/class/majors/tutorialnotefiles/limiting.htm
https://www.khanacademy.org/science/chemistry/chemical-reactions-stoichiome/limiting-reagent-stoichiometry/v/stoichiometry-limiting-reagent
Friday, December 4, 2015
Stoichiometry
This unit was over stoichiometry which is a section of chemistry that involves using relationships between reactants and/or products in a chemical reaction to determine desired quantitative data. That is the literal meaning but it is just the use of reactants or products to determine quantities among them. It is fairly easy since it is mostly conversions. Last time we did conversions I did really well on it and hopefully it wont be any harder than we have seen it to be today.
Here are some links to help with it.
https://www.khanacademy.org/science/chemistry/chemical-reactions-stoichiome
http://chemwiki.ucdavis.edu/Analytical_Chemistry/Chemical_Reactions/Stoichiometry_and_Balancing_Reactions
http://www.chem4kids.com/files/react_stoichio.html
Here are some links to help with it.
https://www.khanacademy.org/science/chemistry/chemical-reactions-stoichiome
http://chemwiki.ucdavis.edu/Analytical_Chemistry/Chemical_Reactions/Stoichiometry_and_Balancing_Reactions
http://www.chem4kids.com/files/react_stoichio.html
Thursday, December 3, 2015
Comments proof
No I don't have to rely on others to print out my comments. Shutout to Eric for giving me the idea. Thanks buddy!
Chemical Reactions Unit test
Today we took the unit test over Chemical Reactions. It wasn't extremely difficult, but there were questions that were fairly difficult. I really can't tell how I did. It can either be really good or really bad. I hope it is really good since my grade is at a good place right now. Hopefully for the next unit I can build on the topics I learn and study to get a higher grade.

Chemical Reactions Lab
Last week we did a lab where we performed a number of different chemical reactions. We did it to determine if precipitates would form. It was practice for the double replacement reaction. We visually got to see the chemical changes that were going off and the change of the solutions to produce the new product was the proof that the reaction occurred. This lab was really cool and fun. I enjoyed doing it and it really helped me understand double replacement reactions.
Metals Lab
This week we did a lab in which we were reacting a metal with some other substance. This lab was used to help us understand single replacement reactions. It helped me understand it since it was many problems that seemed like they would be a topic on the test. This was a very interesting and fun lab to do. I hope we have more like this. We now have to prepare for the test on Thursday. Hopefully it isn't too hard.
Here are some links to help:
Chem med
http://www.chemteam.info/Equations/SingleReplacement.html
https://www.khanacademy.org/science/chemistry/chemical-reactions-stoichiome/balancing-chemical-equations/v/balancing-chemical-equations-introduction
http://chemwiki.ucdavis.edu/Analytical_Chemistry/Electrochemistry/Redox_Chemistry/Oxidation-Reduction_Reactions
Here are some links to help:
Chem med
http://www.chemteam.info/Equations/SingleReplacement.html
https://www.khanacademy.org/science/chemistry/chemical-reactions-stoichiome/balancing-chemical-equations/v/balancing-chemical-equations-introduction
http://chemwiki.ucdavis.edu/Analytical_Chemistry/Electrochemistry/Redox_Chemistry/Oxidation-Reduction_Reactions
Tuesday, November 24, 2015
Chemical Reactions quiz
Today we had a chemical reactions quiz. It was over double replacement reactions. It wasn't too difficult, but there was one question in particular which I had trouble with. I knew how to do it, but the problem was that It took so long to do. Most of the quiz was easy it was just that one question that I thought was hard. Hopefully for the unit test I can prepare for questions like that and get them right.
Wednesday, November 18, 2015
Double replacement
Today was day 2 of the new lesson in chemistry. It is safe to say that I don't understand it that much. We have to memorize 11 solubility rules by Friday to do the lab and memorizing doesn't seem that fun. I understood part of the lesson but it sort of overwhelmed me at times. I hope that within the next couple of days I can comprehend the chapter.
These are some helpful links:
Study.com
Khan academy
These are some helpful links:
Study.com
Khan academy
Tuesday, November 17, 2015
Moles lab
In this lab we had to convert the measurements of either molecules, or mass to moles or vice versa. We used the conversion chart to do this.For both the quiz and the test we had to memorize this chart.

It wasn't hard doing that because we did a lot of practice. This lab was one of those practices and it helped me a lot.
It wasn't hard doing that because we did a lot of practice. This lab was one of those practices and it helped me a lot.
Monday, November 16, 2015
Weekly Quiz
The quiz we took last week was fairly difficult. More than 95% of the test was math and that is why it was difficult. I couldn't tell if the answer I got was right or wrong.We had to convert from moles to mass or atoms and use those in problems to figure out number that should go in front of certain substances like water. We also had to use percent composition for it. There wasn't much but this topic was very important for the unit test since we would need to find the empirical and molecular formulas.


Chemistry Test
I am not sure of what to think about this test. It wasn't necessarily super hard but it also wasn't incredibly easy. The time that we were given didn't help since most of the questions were multi-step problems. Finding the empirical formula wasn't hard since I did the worksheets, it was just time consuming. At first I didn't understand empirical formula and how to find it, but chem.tamu.edu helped me with it.

These links also helped me:
chemteam
Chemical Analysis
Empirical Practice
Chemistry Class
Molar Mass
These links also helped me:
chemteam
Chemical Analysis
Empirical Practice
Chemistry Class
Molar Mass
Chlorine Lab
This week we had a chlorine lab in which we calculated the empirical formula of zinc chloride. We measured the mass of a zinc tablet and had it react with chlorine which made Hydrochloric acid. . It was a fun lab since it was my first lab and we got to make a new substance from two different substances.
Thursday, October 29, 2015
Matter and Measurement Test
After the measurement quiz, I studied hard and tried to understand Significant figures. I understood it better but significant figures mixed with conversion was difficult. I had trouble with some problems. I figured them out but I am not 100% sure I got them all right. I am hoping that I did really good. The conversions were difficult because they were a lot of work and a little miscalculation will cause the entire problem to be messed up.
Mole day
We had a party in chemistry on Mole Day. we had to make a mole and I was baffled by the amount of creative ideas that people came up with. There was a wack-a-mole, mole dancer, guacamoley, holy-moley , and many more. It was pretty impressive. My mole was molezilla, but his name is steven. It was a good party and work day.
Matter lab
This lab was more of an activity to strengthen our understanding of matter and physical and chemical changes that take place in matter. Some stations had questions we had to answer whether the change was a chemical change or physical change. Other stations contained substances that had things in common and we had to determine what type of substances they were. This activity helped us understand matter.
Matter and Measurement pre-test
We took a pre-test last week and I did not know much about the topic. I didn't understand what significant figures means and did not know some physical and chemical properties. The test was difficult and that would explain why I got a 51% on the pre-test.
Video on Density
Video on Conversions and Significant Figures
Dimensional Analysis
States of Matter
Homogeneous and Heterogeneous
Classification of Materials
Video on Density
Video on Conversions and Significant Figures
Dimensional Analysis
States of Matter
Homogeneous and Heterogeneous
Classification of Materials
Significant figures
Significant figures was an easy lesson but there are many places to get a question wrong.We have to remember these rules if we want to get a good grade on this test and on future tests because I am sure that this topic will come up in the future multiple times. The rules are:
1. Non-zero digits are always significant
This means that any number besides 0 is significant. ex- 8, 9, 28, 276 etc.
2. Any zeros between two significant digits are significant.
This means that if a zero falls between two non-zero numbers it is significant. ex. 2002, .200003, 1000000000004 etc. all the zeros in these numbers are significant.
3. A final zero or trailing zeros in the decimal portion ONLY are significant.
This means that zeros after a number that is a decimal are significant. ex. 1.2000000, 10.348500, 0.005000 etc.
1. Non-zero digits are always significant
This means that any number besides 0 is significant. ex- 8, 9, 28, 276 etc.
2. Any zeros between two significant digits are significant.
This means that if a zero falls between two non-zero numbers it is significant. ex. 2002, .200003, 1000000000004 etc. all the zeros in these numbers are significant.
3. A final zero or trailing zeros in the decimal portion ONLY are significant.
This means that zeros after a number that is a decimal are significant. ex. 1.2000000, 10.348500, 0.005000 etc.
Thursday, October 1, 2015
Forensic Archeology Lab
This lab reminded me a lot about what we recently did in Human body systems. In this lab we had to use radioactive dating to determine the age of a skeleton. In HBS we had to find out who the person is using the information given. I enjoyed the concept of the lab but didn't enjoy cutting the pieces of the paper out. It was very tedious. Using the formula and information given we were able to determine how old the bones approximately are and who it was.\
Unit Test
We had our Unit test over Atomic structure and Radioactivity. There weren't many difficult questions but there were some that were time consuming. I knew how to answer every question. If i get something wrong then it was because I didn't work it out correctly. I feel like I got a very good grade on this test,. This unit was pretty interesting but it was harder than the last unit.

http://www.clipartpanda.com/categories/radioactivity-20clipart
http://www.clipartpanda.com/categories/radioactivity-20clipart
Friday, September 25, 2015
Beanium Lab
We recently did the beanium lab. It is an activity to help understand isotopes. Isotopes are elements with same proton count but different neutron count. There were different types of beans and each bean had a different mass. These beans represented isotopes. We used these isotope masses to find out the average atomic mass of the beans. This is a way to help us understand how to do this with different elements and their isotopes.

Atomic Structure and Radioactivity Weekly Quiz
I was well prepared for this quiz. This quiz was over the atomic structure. I understood how to find mass when number of protons and neutrons are given. I also knew who discovered what about the atom. Dalton discovered that the atom was like a solid ball. J.J Thompson discovered that there are electrons in the atom. Rutherford discovered that there is a nucleus in the center of an atom. I also learned how to find the average atomic mass of a substance.
Atomic structure
History
Structure of Atom

http://www.scienceclarified.com/As-Bi/Atomic-Theory.html
Atomic structure
History
Structure of Atom

http://www.scienceclarified.com/As-Bi/Atomic-Theory.html
Saturday, September 19, 2015
Lab
I n class we recently did a lab. This lab we did was a guess the shape inside a little circular can. There was a ball inside and we had to feel around with the ball to try and guess the shape. It was fairly hard but I was able to figure out a couple. Most people didn't get many and overall is was an easy lab to do but guessing was a little difficult.

http://faqb2b.blog.com/ball-bearing-faq/

http://faqb2b.blog.com/ball-bearing-faq/
Friday, September 18, 2015
Pre-Test
We took a pre-test on Atomic theory and radioactivity. I did not understand anything in the test and probably didn't get over a 20 percent. The only thing I sort of knew was half life. I probably got those right. I hope I can learn everything that was on that test and ace the unit test and any quizzes.
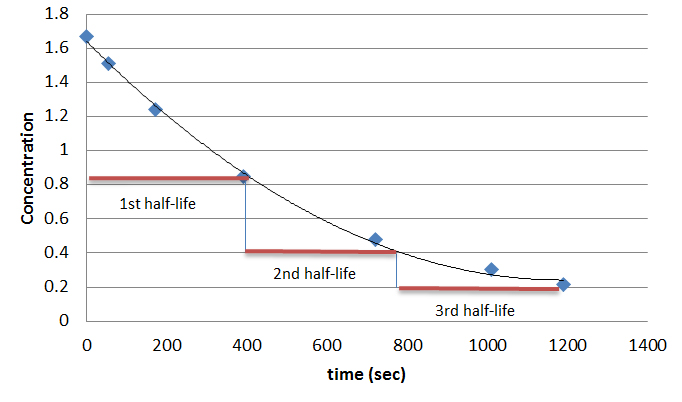
http://chemwiki.ucdavis.edu/Physical_Chemistry/Kinetics/Reaction_Rates/Half-lives_and_Pharmacokinetics

http://chemwiki.ucdavis.edu/Physical_Chemistry/Kinetics/Reaction_Rates/Half-lives_and_Pharmacokinetics
Monday, September 14, 2015
Reflection on Nomenclature 2
Nomenclature was an overall easy unit. Many of the rules about the different type of binary compounds along with polyatomic ions and acids all came relatively easy to me. For studying all i did was go to the online resources that were on schoology and went through the in class notes. Because of this, the lessons and tests were simple, but the Frontier Chemistry project was difficult. It was hard finding all the information and anticipating the questions that would come up on the essay. This is only the first project of the year and it made me a little wary of all the other projects.
Reflection on Nomenclature
Type 1 Binary Compounds
Definition
Type 1 Binary Compounds are compounds that are made up of 1 metal and 1 nonmetal. It was the easiest to name out of all the other types of compounds. It is basic with the metal going first and the non metal going after it. There would be an -ide added as a suffix to the anion which is a nonmetal. The metal ion is called a cation.
Thursday, August 20, 2015
Introduction Page
Subscribe to:
Posts (Atom)
























