Friday, September 25, 2015
Beanium Lab
We recently did the beanium lab. It is an activity to help understand isotopes. Isotopes are elements with same proton count but different neutron count. There were different types of beans and each bean had a different mass. These beans represented isotopes. We used these isotope masses to find out the average atomic mass of the beans. This is a way to help us understand how to do this with different elements and their isotopes.

Atomic Structure and Radioactivity Weekly Quiz
I was well prepared for this quiz. This quiz was over the atomic structure. I understood how to find mass when number of protons and neutrons are given. I also knew who discovered what about the atom. Dalton discovered that the atom was like a solid ball. J.J Thompson discovered that there are electrons in the atom. Rutherford discovered that there is a nucleus in the center of an atom. I also learned how to find the average atomic mass of a substance.
Atomic structure
History
Structure of Atom

http://www.scienceclarified.com/As-Bi/Atomic-Theory.html
Atomic structure
History
Structure of Atom

http://www.scienceclarified.com/As-Bi/Atomic-Theory.html
Saturday, September 19, 2015
Lab
I n class we recently did a lab. This lab we did was a guess the shape inside a little circular can. There was a ball inside and we had to feel around with the ball to try and guess the shape. It was fairly hard but I was able to figure out a couple. Most people didn't get many and overall is was an easy lab to do but guessing was a little difficult.

http://faqb2b.blog.com/ball-bearing-faq/

http://faqb2b.blog.com/ball-bearing-faq/
Friday, September 18, 2015
Pre-Test
We took a pre-test on Atomic theory and radioactivity. I did not understand anything in the test and probably didn't get over a 20 percent. The only thing I sort of knew was half life. I probably got those right. I hope I can learn everything that was on that test and ace the unit test and any quizzes.
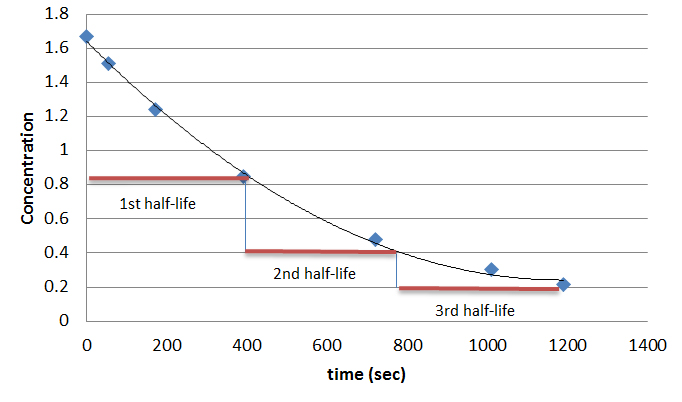
http://chemwiki.ucdavis.edu/Physical_Chemistry/Kinetics/Reaction_Rates/Half-lives_and_Pharmacokinetics

http://chemwiki.ucdavis.edu/Physical_Chemistry/Kinetics/Reaction_Rates/Half-lives_and_Pharmacokinetics
Monday, September 14, 2015
Reflection on Nomenclature 2
Nomenclature was an overall easy unit. Many of the rules about the different type of binary compounds along with polyatomic ions and acids all came relatively easy to me. For studying all i did was go to the online resources that were on schoology and went through the in class notes. Because of this, the lessons and tests were simple, but the Frontier Chemistry project was difficult. It was hard finding all the information and anticipating the questions that would come up on the essay. This is only the first project of the year and it made me a little wary of all the other projects.
Reflection on Nomenclature
Type 1 Binary Compounds
Definition
Type 1 Binary Compounds are compounds that are made up of 1 metal and 1 nonmetal. It was the easiest to name out of all the other types of compounds. It is basic with the metal going first and the non metal going after it. There would be an -ide added as a suffix to the anion which is a nonmetal. The metal ion is called a cation.
Subscribe to:
Posts (Atom)