
Wednesday, May 4, 2016
Review for quiz
We have a quiz tomorrow. I hope it won't be too bad. I am gonna study my notes and do some of the practice tests that she has posted. But here are some websites that might help


Tuesday, May 3, 2016
Avagadro's law
The last law we learned this week is Avagadro's law. This law states that equal volumes of all gases, at the same temperature and pressure, have the same number of molecules. For a given mass of an ideal gas, the volume and amount (moles) of the gas are directly proportional if the temperature and pressure are constant.
 Avagadro's law
Avagadro's law
 Avagadro's law
Avagadro's law
Charles's Law
The next law we learned was Charles's law. This law describes how gases tend to expand when heated. A modern statement of Charles's law is. When the pressure on a sample of a dry gas is held constant, the Kelvin temperature and the volume will be directly related. This is also a little easy to remember.
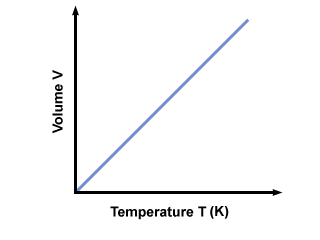
Charles law

Charles law
Monday, May 2, 2016
New Unit
We started a new unit this week and it is about gas laws. most of it is math so it isn't that difficult for me. Remembering the formulas might be a little tricky though so I hope I can get that down.
We have to know each law. the first one is Boyle s law. it just states the relationship between pressure and volume .

crash course
We have to know each law. the first one is Boyle s law. it just states the relationship between pressure and volume .

crash course
Sunday, April 24, 2016
Preparation for test
This unit is pretty easy. I understand it fairly well and have high hopes for it. Here are some helpful links for it.
Energy and Phase Change 1
Energy and Phase Change 2
Energy and Phase Change 3
Energy and Phase Change 4
Mr. Anderson
Crash Course



Energy and Phase Change 1
Energy and Phase Change 2
Energy and Phase Change 3
Energy and Phase Change 4
Mr. Anderson
Crash Course



Specific heat lab
We have a new unit and this is the lab for it. In this lab we tested specific heat. We tested the temperature of our tap water, then boiled our copper in a test tube in a hot water bath. It was a short lab and wasn't my favorite. It was a little fun though.


Friday, April 8, 2016
Building a Boat
Another part of the bio diesel unit is building a boat and racing it. Sadly I wasn't here in the building a boat part but I did help my partner in making the fuel. It is made from oil from chick fila. It was pretty cool making it. I really enjoyed this unit. Our boat did not win 1st, 2nd, or 3rd however. we got 4th. Our class i think had the boat with the longest time though. It was like 2 mins and 40 second. Props to Ganon and Riley for that achievement.


More Info:
http://biodiesel.org/
http://www.afdc.energy.gov/fuels/biodiesel.html
https://www.fueleconomy.gov/feg/biodiesel.shtml
http://www.biodiesel.com/
http://www.biodiesel.com/biodiesel/benefits/


More Info:
http://biodiesel.org/
http://www.afdc.energy.gov/fuels/biodiesel.html
https://www.fueleconomy.gov/feg/biodiesel.shtml
http://www.biodiesel.com/
http://www.biodiesel.com/biodiesel/benefits/
Tuesday, April 5, 2016
Biodiesel Video
Our bio-diesel video and I thought it turned out really well. We got a good idea and made it work. I liked a lot of the video's other people made. They were really interesting and some of them were really funny.
Here is a link to ours: Biodiesel video
Watch it plz

Here is a link to ours: Biodiesel video
Watch it plz

Monday, March 28, 2016
Biodiesel Project
We recently started a bio diesel project. We have to make a PSA that promotes bio diesel and it seems pretty fun. Although, it is pretty hard to come up with an idea. We don't really know what we are doing and right now are just trying to come up with a good idea. The best one is the one like Sarah McLachlan's commercial with animals.




Saturday, March 26, 2016
Unit Test
This unit was by far the hardest for me all year. I had trouble understanding how the atoms would look like and then drawing them out. There were so many things to remember that I got confused a lot. It would not be surprising if I got under an 80 on this r=test. Hopefully the next unit will come to me a lot easier than this one has.
Monday, March 14, 2016
Helpful Links to study
I found this unit one of the most difficult units of the year. I hope these links can help you out.
 Bonding 1
Bonding 1
Khan academy
Bonding 2
Bonding 3
Mr. Anderson
Crash Course
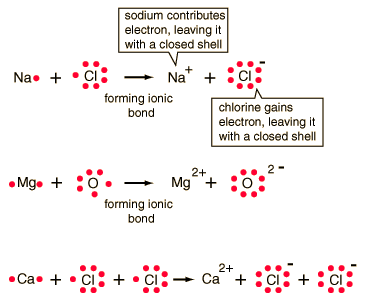
 Bonding 1
Bonding 1Khan academy
Bonding 2
Bonding 3
Mr. Anderson
Crash Course

Saturday, March 12, 2016
New Unit
We stared a new unit and i don't think it will be that hard. However, I have been wrong before on multiple occasions about these type of things. It was just mainly over how the atoms are placed and drawn out in the molecular level. I liked what we did in the library with the physical models. Those are a little more helpful when trying to draw the molecules out.



Monday, March 7, 2016
Unit Test
We recently took the unit test and it was not the hardest of the year. I am feeling pretty confident on it so I hope I did good on it. it was over electronic structure and periodic trends and was fairly easy to understand compared to other chapters. I hope I am as confident about future tests as I am with this one.
Thursday, March 3, 2016
Review for test
This unit has not been difficult, but if not prepared this test can be very difficult just like all other tests. Our test is tomorrow so it would be a great idea to study up. Here are some links to help with the test.
Khan academy
Periodic trends
Chemwiki
chemguide
chemed
Electronic structure
Khan academy
Periodic trends
Chemwiki
chemguide
chemed
Electronic structure
Sunday, February 28, 2016
Weekly Quiz
This quiz wasn't that hard. I thought it was easy and I hope that there are more quizzes and tests like this. I studied hard the previous night so that helped a lot. I am going to make sure I study and prepare for future tests and quizzes like how I did for this quiz.
Thursday, February 25, 2016
Electronic structure and wavelengths Review
So far this chapter isn't too hard. We learned about the different types of ways to map out an electron in the periodic table. Also we learned about wavelengths. we had to know the formulas for wavelengths and energy. The calculation parts are not to hard. After memorizing and doing some problems I knew it wouldn't be too hard.
Wednesday, February 24, 2016
Spectroscopic Analysis
In this lab we had to analyze a cobalt and a chromium compound in order to find their absorbance of light using the machine. It was a fairly easy lab to understand and do. However, the machine was very annoying since the knobs were very touchy and it was difficult to get the calibration we wanted. Other than that it was a straight forward lab.
Friday, February 19, 2016
Flame Test
In this lab, we were burning different chemicals with a Bunsen burner to see what colors were produced. The colors created were pretty cool to look at. Some colors would have to be seen through a cobalt glass since it would cancel out the colors we saw.
Friday, February 12, 2016
Acids and Bases Unit Test
We took our unit test for acids and bases. When Mrs. Frankenberg told us that this test is going to be the hardest test of the year, I was afraid. Therefore I studied a lot and found links online along with the practice sheets that were online. The titration practice helped me a lot. There were a couple of questions on this topic. If I didn't do that worksheet I probably wouldn't feel as comfortable as I do right now. The only mistakes I might have done are calculation problems since there are a lot of chances to miss those.
Wednesday, February 10, 2016
Preparation for Unit test
This unit we are going over Acids and Bases. It is supposed to be the hardest test of the year, but I feel i can do good on it. We need to know how to find PH and find concentration when given PH. The titration problems seem pretty new so I am going to study those and also will review some of the stoic problems because it is used in this chapter. Here are some links to help with these problems.
Acids and Bases Review
Titration
Acid Base
Acid Base 2
Acid Base 3
Equillibrium
Acids and Bases Review
Titration
Acid Base
Acid Base 2
Acid Base 3
Equillibrium
Weak Acid Problems
We learned about how to find a PH and also learned how to find the PH of a weak acid or base. It wasn't too difficult, it was just a lot of work.
This is how you do it:
Video
This is how you do it:
Video
Tuesday, February 9, 2016
Acid lab day 2
We finished our titrations today. We got a pretty light color and a little darker of a pink. However, before that drop the solution was clear but afterwards it was a darker pink so we approximated the last number. That was the hardest part of the titration.
Monday, February 8, 2016
Acid Lab
Today we did a new lab. It was the acetic acid lab. This lab was probably my favorite lab of the year so far or one of my favorite lab of the year. We had to perform titration using KHP, NaOH, and vinegar. At first it was really hard to do since we had to get the solution to change to pink and it had to be ligjht pink. If we add too much base, it would turn dark pink and that was not what we wanted. We had to do this many times over many days and I did enjoy doing it most of the times. This day was just practice for the real lab that we do tomorrow.
Monday, January 25, 2016
Vitamin C Lab
Today we started a lab in class using different juices to determine the vitamin C content in the beverages. We tested pear, apple, v8, a standard, and others to determine this. My partner and I still have other tests to finish up. Then we will determine which of the beverages have the highest amount of vitamin c.
Friday, January 22, 2016
Unit Exam
After that first quiz results, I was worried about chemistry this quarter. However, I prepared for this test in excess and the snow day helped with the preparation. I was able to do a lot more practice problems and it helped me prepare for this test. I feel confident about it and hope I can get more tests where I am this confident about it.
Wednesday, January 20, 2016
Stoichiometry review
Stoichiometry is the a section of chemistry that involves using relationships between reactants and/or products in a chemical reaction to determine desired quantitative data. We use one set of data to transfer information and find out how much is used of the other substance. This is important to know since this chapter uses it in some molarity problems.


Friday, January 15, 2016
Weekly quiz
We took a quiz at the beginning of the week and I thought it was pretty easy. But my scores did not represent that. I didn't do as well as I thought I would. It was over Molarity and I didn't get it all that well. I Understood the saturation graphs but the math and some of the other type of problems troubled me. I will study these topics more and get a better grade on the unit test. Here are some links to help with this chapter.
http://www.chemteam.info/Solutions/Molarity.html
http://www.chemteam.info/Solutions/Molarity-probs1-10.html
http://chemwiki.ucdavis.edu/Inorganic_Chemistry/Reactions_in_Aqueous_Solutions/Unique_Features_of_Aqueous_Solutions
http://www.gcsescience.com/m28.htm
http://www.sciencegeek.net/Chemistry/taters/Unit6SolutionConcentration.htm
http://www.chemteam.info/Solutions/Dilution.html
http://www.chemteam.info/Solutions/Molarity.html
http://www.chemteam.info/Solutions/Molarity-probs1-10.html
http://chemwiki.ucdavis.edu/Inorganic_Chemistry/Reactions_in_Aqueous_Solutions/Unique_Features_of_Aqueous_Solutions
http://www.gcsescience.com/m28.htm
http://www.sciencegeek.net/Chemistry/taters/Unit6SolutionConcentration.htm
http://www.chemteam.info/Solutions/Dilution.html
Subscribe to:
Posts (Atom)













